Updated version of Rice University’s DIY ventilation unit gets FDA emergency use authorization
A refined version of the do-it-yourself ventilation unit originally designed by undergraduate engineering students at Rice University and further developed by the Houston manufacturer Stewart & Stevenson Healthcare Technologies has won emergency use authorization by the U.S. Food and Drug Administration (FDA).
The Apollo ABVM, an automated bag valve mask device, has been approved as an emergency resuscitator for COVID-19 patients in need of a ventilator. Intended as a bridge device for patients who cannot access a traditional ventilator, the programmable Apollo ABVM supplies air into the lungs in a continuous manner until a traditional ventilator is available.
The device, originally created at Rice University’s Oshman Engineering Design Kitchen (OEDK) by undergraduate students, was called the ApolloBVM. But after the COVID-19 pandemic created a global need for medical ventilation alternatives, engineering experts at the OEDK and a Rice undergraduate student joined forces with Rohith Malya, M.D., an assistant professor of emergency medicine at Baylor College of Medicine and an adjunct assistant professor of bioengineering at Rice, to further refine the unit. Instructions for the device are available for download on the Rice University website so that it can be built anywhere in the world—for parts totaling less than $300. According a Rice University news release, the plans have been accessed nearly 3,000 times by individuals in 115 different countries.
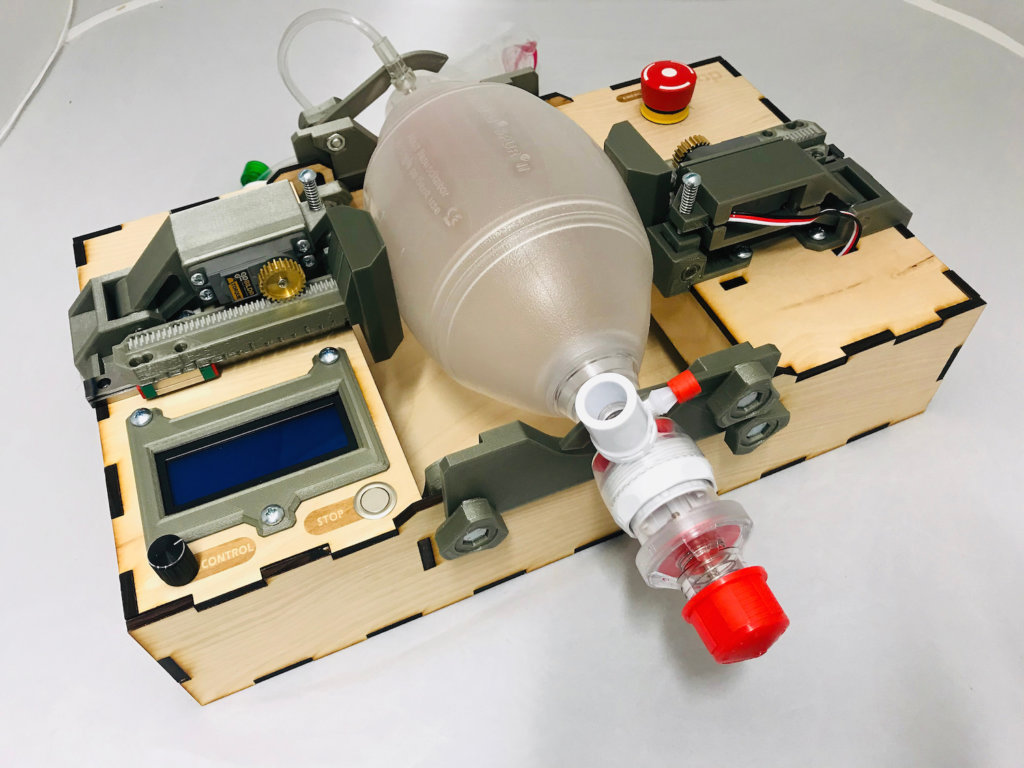
The original device called the Apollo BVM was created by Rice University undergraduates.
“We were hearing that there was a crisis, and there was a challenge that needed to be solved,” said OEDK Executive Director Amy Kavalewitz in an interview about the device in April of this year. “…We say all the time that if this device helps one person survive, then every bit of this effort was worth it.”
But the device’s lifespan wouldn’t end there. The design was picked up for manufacturing by Stewart & Stevenson, which enhanced the DIY version into a sturdier system intended to be easily portable for emergencies. According to a news release put out by Stewart & Stevenson, the device “was refined by S&S to meet the standard for FDA emergency use authorization and make it ready for high volume production.”
The FDA emergency use authorization, which allows for the use of unapproved medical products during emergencies to treat life-threatening diseases or conditions when there are no available alternatives, will last for the duration of the emergency circumstances caused by the COVID-19 pandemic.
Joe Reniers, president of Kirby Distribution and Services, of which Stewart & Stevenson is a subsidiary, said in the news release that the emergency use authorization was an important milestone and that the company “can now commence manufacturing and distribution of this low-cost device to the front lines, providing healthcare professionals with a sturdy and portable ventilation device for patients during the COVID-19 pandemic.”